Digital Inhaler Interest Among Asthma and COPD Patients:
Severity, Anxiety and the Search for Control
In December 2018, the U.S. Food and Drug Administration approved the first digital inhaler for asthma and COPD management, giving patients the ability to capture inspiratory data through a companion mobile app and identify trends over time.
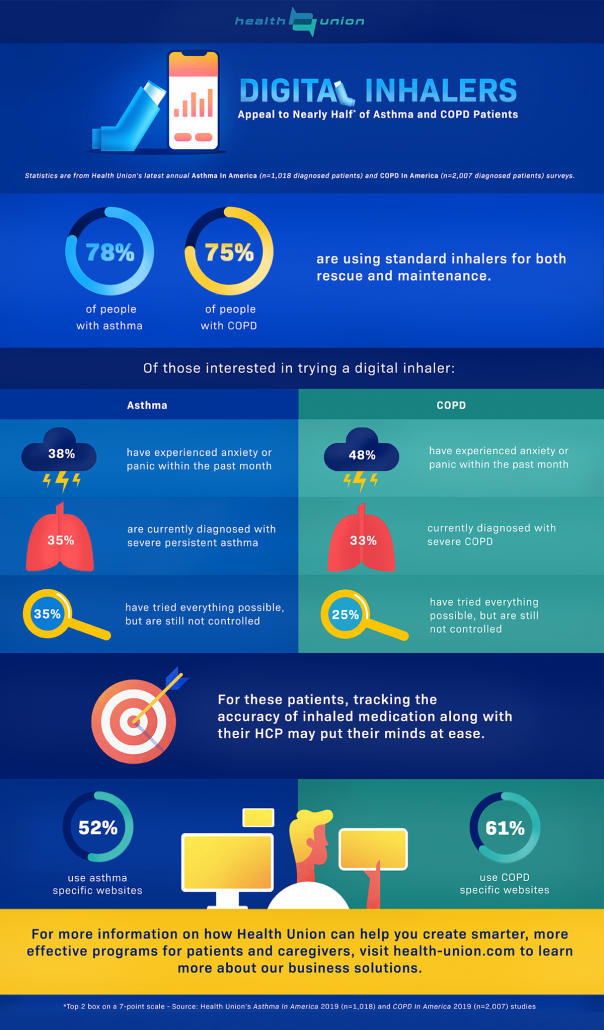 Results of Asthma In America 2019 and COPD In America 2019, syndicated research studies from Health Union, show that many people living with a respiratory disease are already using standard inhalers, with 78% of asthma respondents and 75% of COPD respondents using both rescue and maintenance. Nearly half* of both surveyed populations are highly interested in using a digital inhaler; how can pharma and other healthcare professionals find and meaningfully engage with patients that are likely to give this new technology a try?
Results of Asthma In America 2019 and COPD In America 2019, syndicated research studies from Health Union, show that many people living with a respiratory disease are already using standard inhalers, with 78% of asthma respondents and 75% of COPD respondents using both rescue and maintenance. Nearly half* of both surveyed populations are highly interested in using a digital inhaler; how can pharma and other healthcare professionals find and meaningfully engage with patients that are likely to give this new technology a try?
Data from Asthma In America 2019 (n=1,018) and COPD In America 2019 (n=2,007) show that patients with certain characteristics may be more likely to make the switch. Of the populations surveyed, those interested in using a digital inhaler are more likely to be currently diagnosed with severe persistent asthma or severe COPD than those that are not interested; on top of a severe disease state, they are more likely to be diagnosed with anxiety or panic disorders in addition to their respiratory disease. Respondents interested in using a digital inhaler are also more likely to feel like they have tried everything possible to manage their symptoms, but they are still not controlled.
For the subset of patients living with asthma or COPD that fall into the categories outlined above, the idea of tracking the accuracy of their inhaled medication along with their HCP provides a sense of control. Many survey respondents that are interested in using a digital inhaler also noted that they’d like more insight into their overall treatment and progress, and felt that having more information is useful now and in the future.
Unsurprisingly, both asthma and COPD survey respondents that are interested in using a digital inhaler are more likely to use mobile apps to learn about or manage their condition than those that are not interested. Additionally, this subset of patients living with asthma are more likely to use social media to learn about or manage their condition, and those living with COPD are more likely to utilize COPD-specific websites.
Across both groups, those that are extremely interested in using a digital inhaler are also more likely to be interested in participating in future clinical trials than those that are not interested; this subset of patients is curious about new technologies and treatment opportunities, and looking to find the best solution for their specific characteristics and symptoms.
Although nearly half* of the asthma and COPD survey respondents expressed interest in using a digital inhaler, the remainder expressed moderate or no interest. Across both populations, many that were not interested noted they were concerned about privacy issues. Many COPD respondents were concerned about the cost of the digital inhaler; others noted they were simply not interested.
Survey results offer valuable insight, among all subsets of the populations studied, into the characteristics and behaviors that motivate one to use, or not use, a digital inhaler. Through Asthma.net and COPD.net, marketers can understand, reach and engage people living with these conditions in transparent ways.
Learn more about how Health Union can help you create smarter, more effective programs for patients and caregivers through business solutions in media/advertising, marketing research, and clinical services.
*Top 2 box on a 7-point scale.



