Patient Centricity Starts With Patients:
Closing the Trial Recruitment Gap

Olivier Chateau CEO and co-founder
By Olivier Chateau
CEO and co-founder, Health Union
June 27, 2018
There is a lot of talk in the industry about patient centricity, but is it the reality?
We talk about putting the needs of patients first and keeping them central in the business decisions we make across the healthcare industry. However, as we discussed on a panel at this year’s DIA 2018 Global Annual Meeting, somehow in the effort to amplify patient centricity, a critical voice is often underrepresented—the voice of the patient.
In the clinical trial world, a significant number of trials are delayed or terminated early due to unmet recruitment and enrollment goals. A review of initiated phase two or phase three clinical trials in 2011 found that 19%, nearly 500 trials, were either terminated for failed accrual or were completed with less than 85% of their expected enrollment. Under-enrollment of clinical trials jeopardizes the statistical power and leaves uncertainty when attempting to draw meaningful conclusions from trial results. The question becomes, why aren’t more people participating in clinical trials?
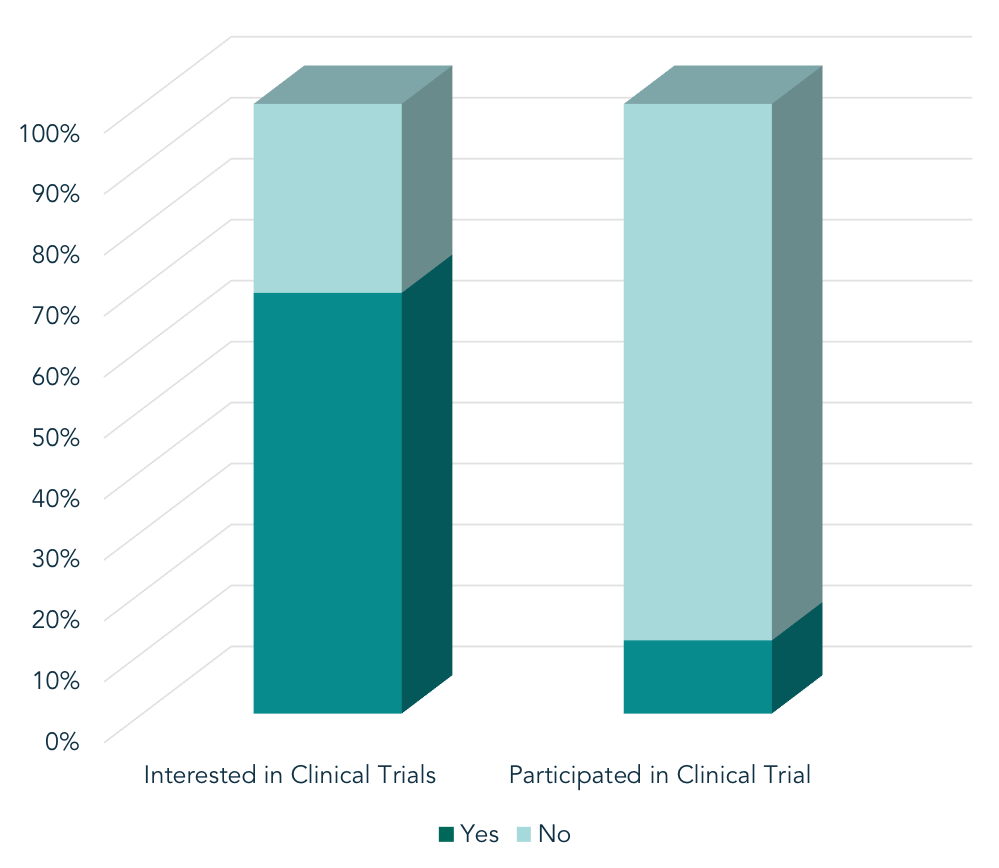
Health Union’s syndicated In America patient surveys reveal a large gap in the percentage of patients who want to participate in clinical trials, and those who do participate. A meta-analysis of proprietary survey data (n = 21,627) revealed a large gap in the percentage of patients who want to participate in clinical trials for their condition, and those who do participate. Of those surveyed within Health Union’s online communities, 69% of patients expressed interest in participating in clinical research. However, only 12% have ever actually participated. What barriers stop those who are interested in trial participation from actually participating?
The perceptions of those who participate in clinical trials and those who merely weigh the decision to participate are often misaligned. To close this gap requires deep understanding of a person’s motivations and employing a patient-centric approach that starts with listening to the patient’s true, authentic voice and understanding the drivers and barriers to participation. Health Union recently surveyed patients who have participated in clinical trials to better understand their experiences, learn more about their motivations, and assess their perceptions of patient-centricity in clinical trials.
While there is often a focus on understanding barriers to trial enrollment and why people don’t participate, what can be learned from those who are interested and make the commitment? The survey revealed that only 53% of respondents were asked by the study team why they were interested in study participation. While we may not know, or address every barrier, learning more from those who actually participate may be the key to understanding how to increase interest and involvement.
Interestingly, while a large percentage of patients participate for their own personal benefit, 70% also cite the benefit to other patients as a motivation. Widely observed throughout Health Union’s online communities, no one better understands the frustrations, pain, and emotional aspects of living with a chronic disease than fellow patients. There is a strong desire among these patients to help “one of their own” and use their own experiences to benefit others. For conditions with hereditary risk factors, patients may be motivated to participate as a way to offer better treatment options and solutions for future generations.
Even when a patient’s motivation for participating is clear, how can we connect those patients who are interested with trials that are right for them? Only 32% of patients noted their doctor’s recommendation as a reason for participation. With limited time at each appointment, patient/doctor conversations about clinical trials may not be occurring. Trial staff and industry sponsors should find ways to help facilitate these conversations and help interested patients learn about clinical trials as a care option. While the trial process is confusing to many, surveyed patients had a good understanding of the study process, but weren’t often asked what they expected of the trial itself. Opening a dialogue can help to reassure those who are unsure or confused get the answers they need and deserve.
Another way study staff and industry sponsors may be able to increase recruitment success is by improving communication of the trial’s end results. The Health Union survey found that just 30% of respondents were notified of the end results of the study in which they participated. Sharing results from trials offers even more ways to engage with patients, and provides tangible evidence for the patient that validates their motivations to help both themselves and others.
Generating interest in clinical trial programs starts with understanding patient perceptions, awareness and interest in participating. Ongoing communication, including sharing results, makes the experience more patient centric and fills a desire for people to feel like the study was not only useful to them, but to others who are experiencing the same health challenges.